Abstract
Background
Thiopurines 6-mercaptopurine (6-MP) is the critical anticancer agent used for childhood acute lymphoblastic leukemia (ALL) maintenance therapy but the therapeutic window is limited. Previous genome-wide association study (GWAS) had identified that TPMT polymorphism is associated with 6-MP metabolism and ALL patient with low TPMT activities exhibit 6-MP intolerance. However, TPMT variants are extremely rare in the East Asian population, so TPMT polymorphisms alone cannot explain the 6-MP intolerance observed in Asians. Recently, there are several genetic traits which are associated with 6-MP intolerance had been identified in East Asian cohorts including NUDT15, ITPA and MRP4 . In this study, we had examined these genetic polymorphisms and the correlation with 6-MP dose intensity in Taiwanese cohort.
Methods
From January 1 2003 to December 31 2012, total 137 patients diagnosed with childhood ALL were recruited in this study. Recent genome-wide association studies identified many novel genetic variants contributing to host susceptibility to adverse effects of antileukemic agents (e.g., vincristine, asparaginase, glucocorticoids and Mercaptopurine). By literature review, we selected the recently-identified genetic variants to study. Germline DNAs were prepared by standard methods from peripheral blood mononuclear cells. Q-PCR were designed to identify these SNPs. The coding regions of the NUDT15 gene were first amplified by PCR from germline DNA, followed by Sanger sequencing. Clinical data were correlated with these genetic variants.
Results
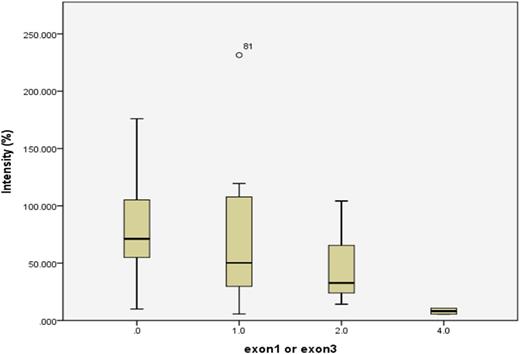
There are four SNPs of NUDT15 identified in this cohort. The most common are p.Val19_Val19insGlyVal (rs554405994) and p.Arg139Cys (rs116855232). Patients with NUDT15 exon1 variant (rs554405994) in standard-risk (SR) and high-risk (HR) groups have Mercaptopurine intolerance in comparison with patients without this SNP in the same groups. On logistic model, P values on the additive model and dominant model were 0.036 and 0.039, respectively. Patients with NUDT15 exon 3 variant (rs116855232) also tolerated less Mercaptopurine dose. The SNPs of ITPA and MRP4 showed no significant impacts on the dose of Mercaptopurine in this cohort.
Conclusions
The coding variants of NUDT15 accounted for Mercaptopurine intolerance in SR and HR patients. It is suggested that genetic analysis should be performed before the administration of Mercaptopurine to avoid the complications of myelosuppression, especially for patients with homozygous variants. GWAS should be performed to discover novel genetic variants contributing to complications of ALL chemotherapy
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal